Hyperthermia and the temperature issue
Without a doubt, hyperthermia as a therapeutic method is about achieving a temperature increase in the patient's body. But then the consensus among clinicians almost stops. And indeed, the application submitted to the joint federal committee in 2005 to have hyperthermia reimbursed as a service provided by the health insurance companies in Germany failed not on the grounds that hyperthermia was not effective, but rather because - quote: "...no medical-scientific consensus has yet been reached with regard to the evaluation of the therapy results and the necessary standardization (e.g. temperature, exposure time, thermometry, accompanying therapy protocols).
Now 15 years have passed. What has happened since then?
An important development concerns the desired temperature. In the past, the higher the temperature reached, the better. This is seen today however much more differentiated. This begins above all with the question of what hyperthermia should achieve in a specific case. Should it make radiotherapy more effective? Should it improve the effect of certain chemotherapies? Is it used primarily to strengthen the immune system of a patient? Different areas of application require different temperature ranges.
Temperature targets
Very high temperatures (>44 degrees C) seem problematic, because over a longer period of time they can cause cells exposed to such temperatures to die. For a tumor cell this may be desirable, but for nerve cells or vital functional areas in organs this may be dysfunctional. Apart from this, it is also technically extremely demanding to reach such temperatures in the depth of a body organism, which is also cooled by continuous blood circulation.
The highest desired temperatures of 41-43 degrees Celsius apply to an application in combination with radiotherapy. Such a high temperature in the regional target area of a tumor is considered advantageous.
To make chemotherapy more effective, a temperature in the range of a natural fever (from 390C to 410C, maximum 420C) seems to be best. Thus, an effective lymphatic intercourse takes place, i.e. a good preponderance of cytostatic drugs into the tumor tissue as well as a metabolism accelerated by the higher temperature (processing in a cell).
In the field of immunotherapy, there are still hardly any studies that can be used for a corresponding evaluation. Apart from special applications, the target corridor is also rather in the moderate range between 390C and 410C.
temperature measurement
The second crucial question is then: how do you make sure that you can achieve the desired temperature?
There are various technological processes for this purpose. In the case of whole-body hyperthermia, a water-cooled InfraRed-A light has proven to be the most suitable (a sauna, by the way, does not lead to the desired result). And here the core body temperature, which is supposed to cover the whole body, can be measured very easily, for example in the intestine.
In local deep hyperthermia, the heating of a region in the depth of the body, almost all providers currently work with electromagnetic energy that is introduced into the body, either via antennas around the body or as a capacitive method, in which the patient is placed between two electrodes whose polarity changes very quickly. However, the question remains: how do you ensure that the desired temperature can be reached?
Quite simply: by measuring the temperature in the target area. But how do you do that if the target area, like most tumors, is inside the body?
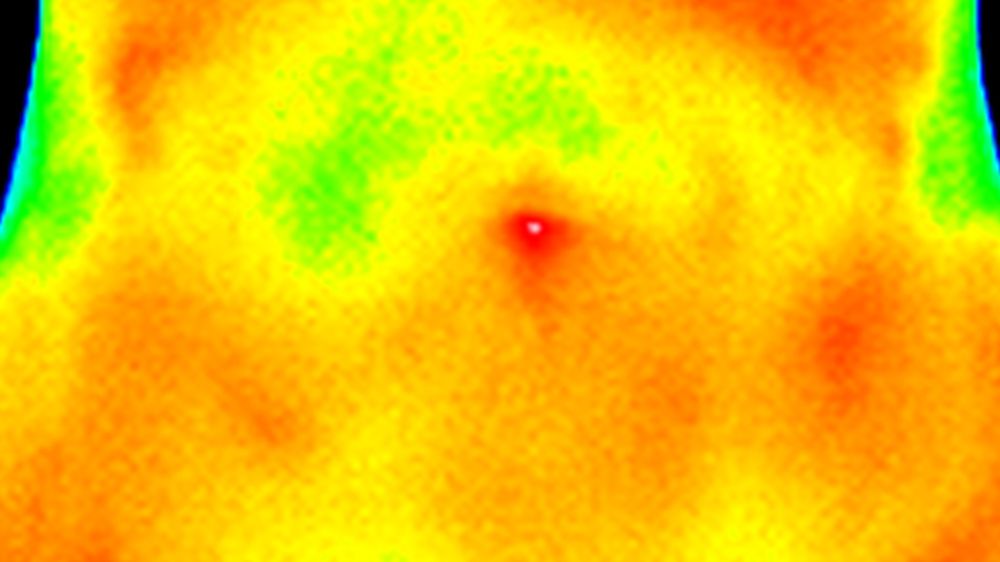
There are good possibilities if the tumor lies in a natural body orifice. For example, in the case of ovarian tumors in women, the tumor can be reached through the vagina, or in the case of rectal cancer, through the anus; the prostate can also be reached either through the urethra or, more approximately, rectally. But even here there are pitfalls, because depending on where the sensor is located at the tip of an optical fiber cable, one may only measure the air in the intestine, which is relatively unaffected by heating by means of electromagnetic fields. However, for the majority of tumors located in the body, it becomes much more problematic. Under image control (ultrasound or x-ray fluoroscopy), one would have to use a hollow needle to prick the tumor and then insert a temperature probe. Even then, the unsatisfactory fact remains that the temperature can only be measured at a single point. What if this point comes to rest right next to a blood vessel? In that case, there will be no significant temperature gradient, because a larger vessel acts like a cooling system on its immediate surroundings. So, to arrive at a more valid assessment, there would actually have to be several measurement points in the tumor target area.
But there is another, very elegant solution. With an MRI, the temperature differences can be measured and displayed spectroscopically or with another method. This has two advantages: Firstly, nothing has to be introduced into the body (and unlike a CT, an MRI does not expose the body to radiation) and secondly, not only can a point measurement be obtained as with a temperature sensor, but entire sectional images can also be obtained in which the temperature distribution in the area can be seen. Unfortunately, there is a decisive disadvantage: such an MRI has to be adapted and can then no longer be used for normal imaging; ergo with several hundred thousand Euros it is simply too expensive for normal use. And so this type of temperature measurement is only available at very few university hospitals in Germany.
As an interim summary: the temperature measurement is not so easy, because who wants to have a hollow cannula inserted into the tumor on a regular basis? And last but not least, every invasive procedure also involves the potential risk of tumor cell transplantation.
Other quality assurance measures
It is understandable that alternative forms are being sought which can compensate or replace a direct measurement of the temperature if necessary.
In some places this is attempted by temperature simulation software, which uses the CT scans, which are usually available anyway, as a starting point. The different tissue types such as muscle, internal organs, bones or lumen can then be differentiated well and since the temperature coefficients of these tissue types are known, simulations can be used to calculate how much energy would have to be introduced into the body for a desired temperature and, above all, how this would be controlled. However, this method also has a decisive handicap, namely the cooling blood perfusion. Especially in tumor tissue, blood perfusion is highly irregular and within an hour it can double or reduce up to three times with a decisive effect on the temperature. Too high a temperature can also be problematic if so-called hot spots appear at important physiological locations.
A pragmatic form of alternative quality assurance for achieving the temperature is the use of standardized therapy performance protocols. If such performance protocols exist differentiated according to different tumor entities for a device and if such performance protocols have been accurately evaluated with phantom measurements and(!) temperature measurements in patients who have voluntarily agreed to this, then clues can be obtained as to which temperatures are reached in which body regions at which performance levels. It remains inaccurate that in a single session the desired temperature may not have been reached - if, for example, the cooling blood perfusion was very high - but on average for a series of applications, there are at least valid indications. Deviations can also occur if a single patient is very obese, for example. In this case, deviations from the protocol are necessary, because fat layers absorb large amounts of energy.
**What does this mean for the application and the patient?
The performance protocols, which are based on a higher temperature target, are demanding! Especially in the last third of a treatment, a specialist is needed who can optimize a treatment at the bedside with the patient. For example, body sweat, which occurs naturally, must always be wiped dry, as the salty sweat can act like a magnifying glass for the electromagnetic energy and then cause painful stinging sensations. There are many small things to consider in order to keep a really demanding treatment pleasantly bearable for a patient. Anyone who makes compromises here and simply reduces the power input is simply not making full use of the potential of this application. Also, a treatment should last about one hour - with the exception of head and neck tumors.
Many clinical studies have shown hyperthermia to have an excellent potential to make treatments more effective and to achieve significantly longer survival times. Also, the ratio of effect to undesirable side effects is excellent when hyperthermia is applied correctly, but it must also be applied correctly.
**Literature
For readers interested in further discussion, please refer to the following literature:
ESHO quality guidelines for surface hyperthermia
Dobšíček Trefná H, Crezee J, Schmidt M, et al. Quality assurance guidelines for superficial hyperthermia clinical trials : II. Technical requirements for heating devices. Quality assurance guidelines for local hyperthermia clinical trials : II. Technical requirements for heating devices. Radiation Heater Oncol. 2017;193(5):351-366. doi:10.1007/s00066-017-1106-0
Series of in-vivo temperature measurements in live pigs to validate a standard protocol for liver treatment with the Celsius42 device
Noh JM, Kim HY, Park HC, et al In vivo verification of regional hyperthermia in the liver. Radiat Oncol J. 2014;32(4):256-261. doi:10.3857/roj.2014.32.4.256
Article about MRT temperature measurement in hyperthermia
Lüdemann L, Wlodarczyk W, Nadobny J, Weihrauch M, Gellermann J, Wust P. Non-invasive magnetic resonance thermography during regional hyperthermia. Int J Hyperthermia. 2010;26(3):273-282. doi:10.3109/02656731003596242
Article about pre-clinical temperature experiments with capacitive hyperthermia (Celsius42 device)
H. Sahinbas, M. Rosch & M. Demiray (2017) Temperature measurements in a capacitive system of deep loco-regional hyperthermia, Electromagnetic Biology and Medicine, 36:3, 248-258, DOI: 10.1080/15368378.2017.1307221
Investigation of the type and extent of oxygen enrichment by regional hyperthermia
Cho CH, Sreenivasa G, Plotkin M, Pietsch H, Wust P, Lüdemann L. Tumour perfusion assessment during regional hyperthermia treatment: comparison of temperature probe measurement with H(2)(15)O-PET perfusion. Int J Hyperthermia. 2010;26(4):404-411. doi:10.3109/02656731003605662
Two general. Reviews on the potential of hyperthermia in clinical tumor therapy
Datta NR, Ordóñez SG, Gaipl US, et al Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742-753. doi:10.1016/j.ctrv.2015.05.009
Datta NR, Kok HP, Crezee H, Gaipl US, Bodis S. Integrating Loco-Regional Hyperthermia Into the Current Oncology Practice: SWOT and TOWS Analyses. Front Oncol. 2020;10:819. Published 2020 Jun 12. doi:10.3389/fonc.2020.00819
